Cancer remains one of humanity’s most formidable health challenges. According to the World Health Organization, over 35 million new cancer cases are predicted in 2050, a staggering 77% increase from the 20 million cases estimated in 2022. This sharp climb underscores the urgency for scientific innovations in cancer research.
Yet, the horizon of oncology is brightened by transformative investigational therapies that promise to reshape patient outcomes. Bringing these breakthroughs from laboratory to bedside requires more than scientific discovery—it demands the operational expertise, regulatory insight, and patient-centric approach of Contract Research Organizations (CROs) like ExperTrials.
In this blog post, we have classified those therapies based on their mechanism within the patient to give you a clearer view of how today’s most promising innovations are changing the future of cancer care. We also explain how CROs, and ExperTrials in particular, are the bridge between scientific innovation and real-world patient benefit. Finally, we present our case study on the glioblastoma trial that we were contracted to conduct in 2012.
Part 1: The Most Promising Cancer Therapies Today
Cancer therapy is undergoing a revolution, driven by breakthroughs that approach the disease from fundamentally different angles, from teaching the body to fight back to acting at the genetic level, changing the patient’s microbiome, or harnessing the power of AI.
Immune-Based Therapies: Teaching the Body to Fight Back
Exciting advances in cancer immunotherapy are empowering a patient’s own immune system to recognize and destroy cancer cells. mRNA-based vaccines, while best known for their role in infectious diseases, are now showing remarkable promise in targeting tumors. These vaccines introduce cancer-specific mRNA sequences, training immune cells to recognize and attack malignant tissue. Recent clinical trials, especially in notoriously difficult cancers like pancreatic cancer, highlight their potential to improve outcomes.
Example: Moderna and Merck are running a Phase IIb trial of an individualized mRNA vaccine (mRNA-4157/V940) in combination with pembrolizumab for melanoma (KEYNOTE-942). Early results showed a reduced risk of recurrence.
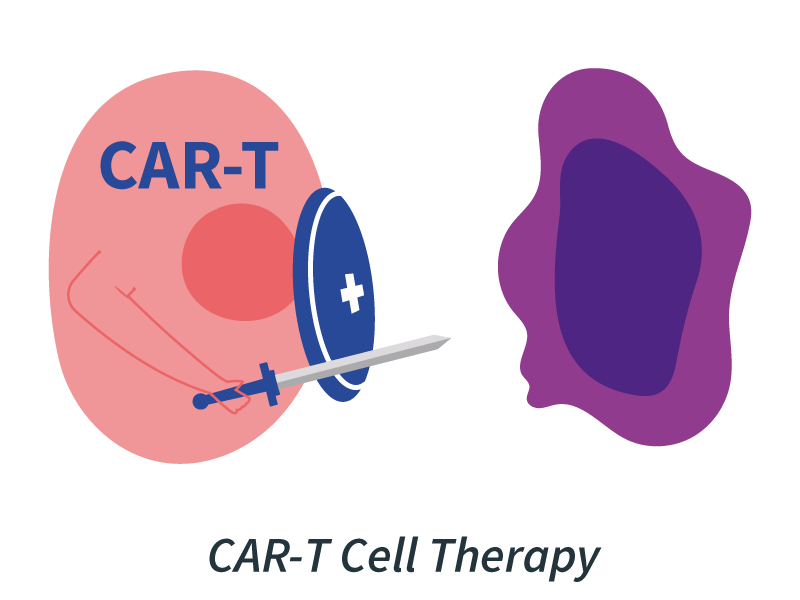
Meanwhile, CAR-T cell therapy takes a more hands-on approach by engineering a patient’s T cells outside the body, reprogramming them to identify and kill cancer cells upon reinfusion. Originally approved for certain blood cancers, CAR-T therapies are now being adapted for solid tumors, aiming to extend their life-saving reach to more patients.
Example: Several Phase I/II trials are testing CAR-T therapies against solid tumors, such as mesothelin-targeted CAR-Ts in pancreatic and ovarian cancer (NCT04581473).
Genomic and Cellular Editing: Precision Redefined
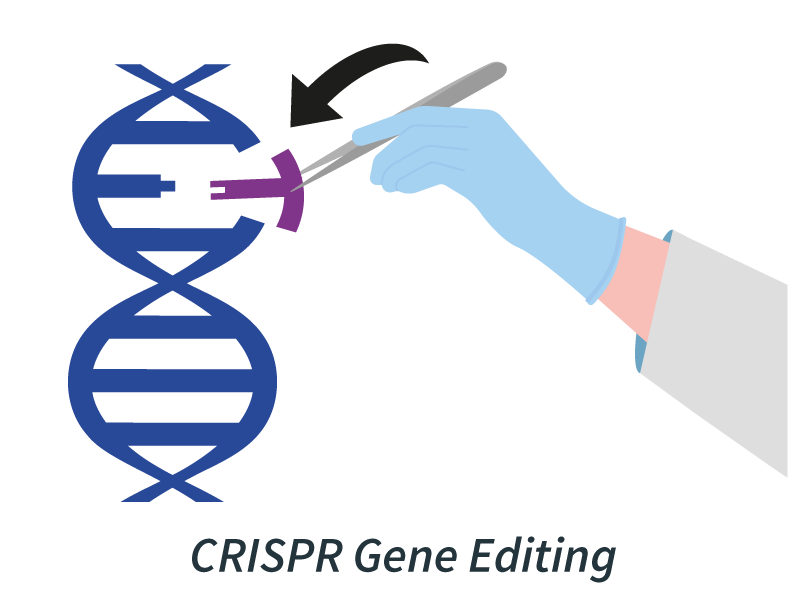
The next frontier in cancer treatment involves making surgical strikes at the genetic level. CRISPR gene editing tools allow scientists to modify DNA with unprecedented accuracy. In oncology, CRISPR is being used to engineer T cells that are even better at homing in on and destroying cancer cells, potentially overcoming some of the limitations of traditional immunotherapies.
Example: The first-in-human CRISPR trial (NCT03399448) edited patient T cells for enhanced activity against refractory cancers such as sarcoma and multiple myeloma.
On another front, KRAS inhibitors tackle one of the most infamous oncogenes directly. KRAS mutations are notoriously difficult to drug, often found in lung, colorectal, and pancreatic cancers. Breakthrough KRAS inhibitors now offer new hope by blocking these mutant proteins, slowing or even reversing tumor growth in patients who previously had few good options.
Example: The CodeBreaK 200 trial (NCT04303780) tested sotorasib in KRAS G12C-mutated non-small cell lung cancer (NSCLC). It demonstrated significant progression-free survival benefit compared to docetaxel.
Microbiome and Molecular Environment: Targeting Context, Not Just Cells
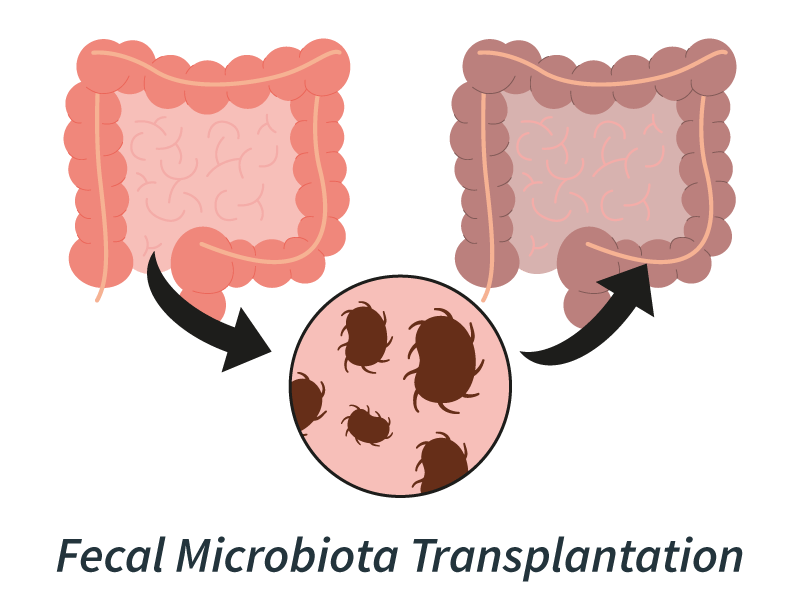
Scientists now appreciate that cancer’s environment can be just as important as the tumor itself. Fecal Microbiota Transplantation (FMT) represents a novel method for modulating a patient’s gut microbiome. Originally developed to prevent graft-versus-host disease in blood cancer stem cell transplants, FMT is now being explored for its potential broader role in oncology, as the gut microbiome has a deep influence on immune function and treatment outcomes.
Example: Dana-Farber Cancer Institute (NCT04116775) is testing whether FMT can restore responsiveness to immunotherapy in patients with advanced melanoma who previously failed PD-1 blockade.
AI & Next-Generation Diagnostics
Finally, advanced technologies powered by artificial intelligence are rapidly accelerating cancer research and precision medicine. Techniques like single-cell analysis and spatial transcriptomics give scientists a chance to see cancer at a previously unimaginable level of detail — understanding not just which cells are present, but exactly what they’re doing, and where. AI integrates this vast data, helping clinicians and researchers target therapies with laser-like accuracy and uncover new pathways for intervention.
The convergence of these approaches is bringing real hope for better outcomes, fewer side effects, and a future where cancer is a far more manageable — or even curable — disease.
Example: At Mayo Clinic, AI-driven radiology studies are improving accuracy in prostate and lung cancer detection by reducing false positives.
Part 2: The Crucial Role of CROs in Cancer Innovation
Contract Research Organizations (CROs) are instrumental in bridging scientific ideas and clinical reality. ExperTrials stands apart from conventional CROs by combining global capabilities with small-company agility and deep expertise:
➡️ Agile, Full-Service Model: Unlike large organizations, ExperTrials provides comprehensive services—clinical operations, regulatory affairs, data management, biostatistics, medical monitoring, pharmacovigilance, quality assurance, and market access—delivered by senior experts only. A lean team that is able to respond rapidly to sponsor needs.
➡️ Global Reach with Local Expertise: Operating across 24 countries, ExperTrials leverages a network of 80+ senior Clinical Research Associates (CRAs) in Europe and the USA, supervised by dedicated leaders like Ghazala Kabani (US) and Elzbieta Lech (EU), ensuring both broad coverage and genuine grassroots engagement.
➡️ Tailored, Strategic Partnerships: ExperTrials functions as an extension of each sponsor’s team. We offer targeted project management, regular transparent communication, and solutions tailored for startups and biotechs, especially those requiring cross-border trials and regulatory navigation in the US and EU.
➡️ Horizontal Leadership and Entrepreneurial Spirit: The company’s management style emphasizes empowerment, flexibility, and ownership, attracting passionate researchers motivated to deliver quality and innovation in every study.
➡️ Optimized Recruitment & Site Engagement: Targeted strategies ensure rapid enrollment and effective retention, harnessing real-world investigator relationships to accelerate timelines and mitigate risks.
➡️ Integrated Regulatory and Quality Oversight: In-house experts ensure compliance with FDA, EMA, and GDPR requirements, offering authorized EU representation and continuous quality improvement throughout the trial lifecycle.
What truly differentiates ExperTrials is its commitment to personalized service, flexible engagement models, and strategic guidance every step of the way. Those assets are crucial for sponsors aiming to launch cancer innovations quickly and globally.

Part 3: ExperTrials’ Case Study — Rescuing a Glioblastoma Clinical Trial
Glioblastoma (GBM) is the most aggressive and lethal primary brain tumor in adults, with a survival rate averaging just 15–18 months and frequent recurrence despite standard care. Recognizing these challenges, ExperTrials was contracted in 2012 to rescue a large international Phase III GBM clinical trial spanning 40 study sites across Europe after sponsor dissatisfaction with a previous CRO.
Our Key accomplishments:
➡️ Rescue and management: Successfully transitioned and completed the trial, upholding data integrity and reigniting recruitment.
➡️ Expanded expertise: Managed additional Phase II/III trials in newly diagnosed/recurrent GBM and other solid tumors for the same device manufacturer.
➡️ Global impact: Supported device registration for GBM treatment in Europe and the USA, navigating FDA post-marketing studies and inspections.
➡️ Oncology leadership: Leveraged regulatory expertise, deep site relationships, and a patient-centric approach throughout study resuscitation.
ExperTrials has also supported other complex CNS and oncology programs such as cerebrolysin in ischemic stroke (Phase II, 80 patients across Poland, Ukraine, Romania) and the ImpACT-24 trial in acute ischemic stroke (Phase III, 450 patients across the US, EU, Hong Kong, Israel).
This case study spotlights the exceptional value a nimble, dedicated CRO like ExperTrials brings to clinical innovation—especially in high-stakes, complex disease areas.
Conclusion
Accelerating access to life-saving therapies hinges on the collaboration of sponsors, CROs, patients, and regulators. Every partner brings vital expertise and engagement. At ExperTrials, our unwavering commitment is to advance cancer care through innovation, operational excellence, and a laser-focus on patient impact. Together, we can translate investigational therapies into new standards of care, providing hope to millions where it’s needed most.
Are you advancing cancer research or clinical trials?
Let’s discuss how ExperTrials can propel your ambitions and help launch life-saving therapies across the globe.