The clinical research landscape is evolving. With the release of ICH E6 (R3), expectations around sponsor oversight and accountability in clinical trials have never been higher. As the updated guideline redefines the boundaries of delegated responsibilities, sponsors can no longer rely solely on the structures of full-service outsourcing or the flexibility of functional service providers. In this context, the way they approach CRO partnerships is decisive.
Should the preference be for the comprehensive coverage of FSO, the agility of FSP, or an innovative hybrid? This blog explores the differences and advantages of FSO and FSP models, how ICH E6 (R3) elevates sponsor obligations, and the unique strengths of the ExperTrials hybrid model.
Part 1: FSO vs. FSP: Which Clinical Outsourcing Model Fits Your Needs?
As clinical trials grow increasingly complex, selecting the right outsourcing model is more strategic than ever for biotech and medtech companies. The two main approaches are Full-Service Outsourcing (FSO) and Functional Service Provider (FSP), each offering unique advantages.
Full-Service Outsourcing (FSO)
FSO is the traditional model where the sponsor delegates all trial-related activities to a CRO. Key features include:
- End-to-end trial delivery, covering everything from study start-up to data management, monitoring, and regulatory services
- Use of the CRO’s SOP framework and quality systems for standardization
- Centralized project management, ensuring predictable processes, but sometimes at the expense of flexibility
- Streamlined access to specialized expertise at every stage.
This model is best suited for sponsors seeking minimal operational burden and a single point of accountability.
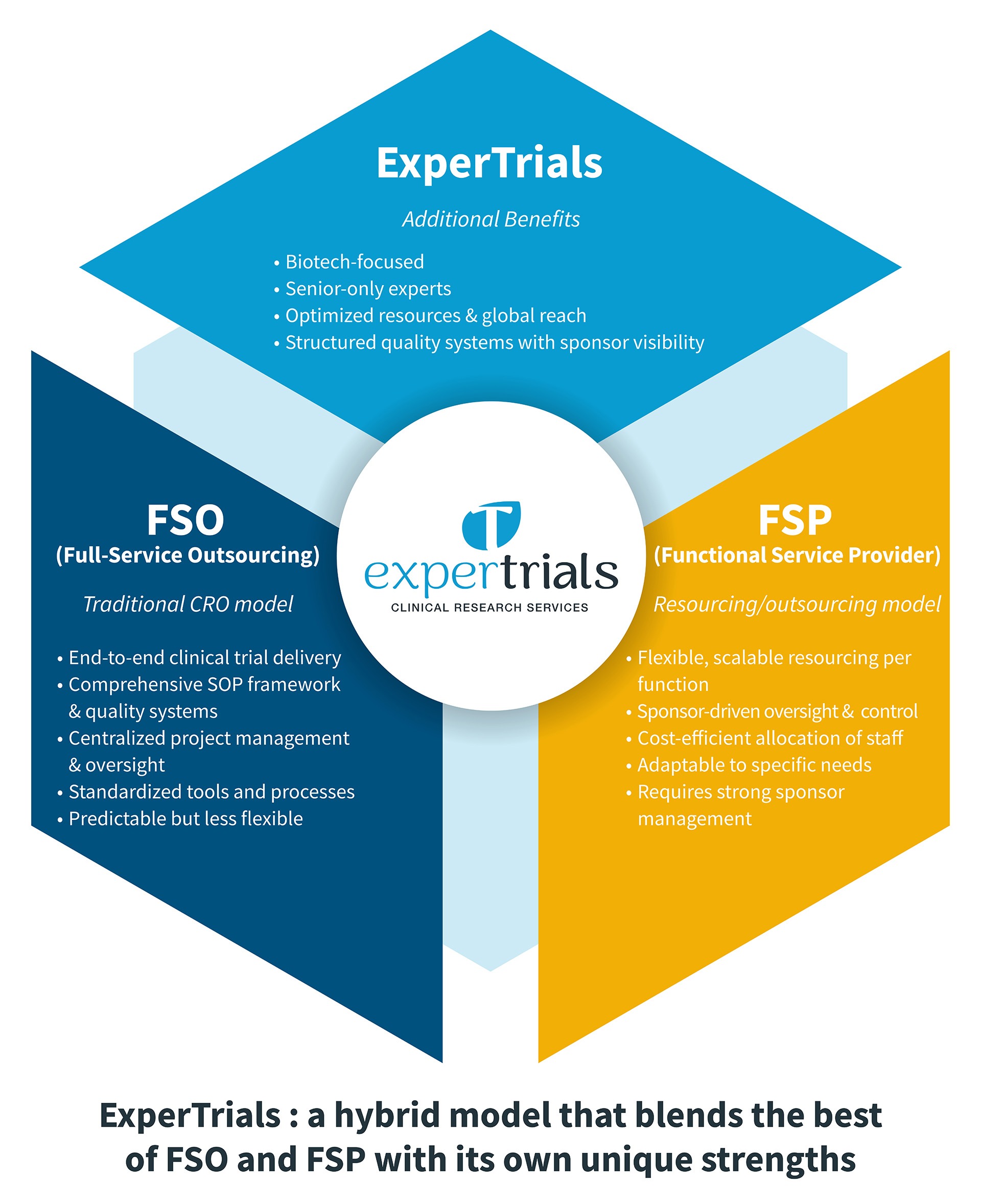
Functional Service Provider (FSP)
FSP allows sponsors to outsource specific functions, such as data management, regulatory affairs, or monitoring, while maintaining overall trial oversight. Here is what this model includes:
- Greater flexibility and scalability to adjust resources per function or study.
- Sponsor-driven oversight and control, promoting transparency and strategic involvement.
- Cost-effective allocation of staff, adaptable to unique project needs.
This model can be more labor-intensive for the sponsor, requiring robust internal management and oversight. Recent industry trends show a surge in FSP adoption, with many companies favoring its adaptability and resource optimization.
Part 2: How ICH E6 (R3) Changes Sponsor Responsibilities
The latest update to Good Clinical Practice, ICH E6 (R3), brings a major shift: sponsors retain ultimate responsibility for the trial, even when delegating activities to a CRO or vendors.
Sponsors must establish comprehensive partner assessment and risk management systems throughout the trial. Oversight plans must be documented and auditable—continuous, proactive supervision is required for all outsourced tasks. It’s no longer enough to rely on the CRO’s reports: sponsors are expected to challenge assumptions, monitor compliance, and act immediately if issues arise.
This new regulatory paradigm demands sponsors choose CROs equipped for both structured process and flexibility—plus clear documentation and real-time visibility.
Part 3: Why ExperTrials’ Hybrid Model Is the Future-Proof Solution
ExperTrials, a small-sized CRO, has developed a hybrid model that blends the strengths of FSO and FSP. This approach aligns perfectly with the new ICH E6 (R3) requirements. Our model guarantees:
- Structured quality systems and operational frameworks, ensuring compliance with both FSO consistency and regulatory expectations.
- Flexible, scalable resource deployment—sponsors can adjust support as projects evolve, maintaining strategic control and visibility at all times.
- Sponsor-driven oversight: an insourced project manager works alongside ExperTrials’ international team of senior clinical experts to ensure transparency and sponsor accountability.
We focus on biotech clients, providing senior-only expert teams, global reach, and optimized resource allocation. By offering this hybrid solution, ExperTrials empowers sponsors to adapt quickly to regulatory demands and industry shifts, all while maintaining full control and compliance.
Are you a biotech ready to thrive under the new ICH E6 (R3) standards?
Let’s discuss how we can optimize your resources, accelerate development timelines, and reduce trial costs while ensuring regulatory alignment!
